
Financial support to this project by the European Union through Next-Generation EU funds (National Recovery and Resilience Plan, PNRR - Mission 4 “Education and Research” - C2 component - Investment 1.1, Fund for the National Research Program and Projects of Relevant National Interest, PRIN). .
Brief description of the project
Water electrolysis (WE) powered by renewable sources is the “greenest” way to produce H2, a promising energy vector that could help bring the World to net zero emissions in the coming decades. The project is focused on the development and engineering of novel nanostructured electrocatalysts for “green hydrogen” production via WE based on high-entropy materials (HEMs). Its ambition is the achievement of cost-effective efficient critical raw materials-free catalysts for the oxygen and hydrogen evolution reactions to be operated in an electrolyser that employs a polymeric anion exchange membrane (AEM) as electrolyte.
Two solution-based scalable techniques, namely sol-gel and electrospinning, will be used for the production of HEMs in the form of nanoparticles and nanofibers. Their preparation conditions will be optimised in order to obtain HEMs for electrode preparation by ink spraying, with suitable properties to enhance the electrocatalytic performance and stability.
HEM4GREEN project will explore the entire value chain including: i) investigation of new electrode materials; ii) fabrication of electrodes for AEM electrolyser; iii) their assembly to obtain 1 A/cm2 current density at 2V potential at 50°C and a single cell degradation rate below 5-7 µV/h at 1 A/cm2 current density, corresponding to about 0.2-0.4 %/1000 h.
- Duration of the project: 24 months
- Strategic emerging topic: Sustainability and projection of natural resources
- Cluster: Climate, Energy and Mobility .

Research units
Investigators
- Saveria SANTANGELO (*) - [PI] - UNIRC
- Sabrina CAMPAGNA ZIGNANI (*) CNR-ITAE


Department of Civil, Energy, Environmental and Materials Engineering (DICEAM)
Mediterranean University of Reggio Calabria
89122 Reggio Calabria
Italy


Institute of Advanced Technologies for Energy (ITAE) of the National Research Council (CNR) - Messina -Italy
Project sector relevance and coherence to strategic themes
The design of cost-effective platinum group metal (PGM)-free nanostructured electrocatalysts with a large surface area and an easily tunable composition is of pivotal importance to overcome the limitations imposed by the sluggish kinetics and large overpotential and, thus, make water electrolysis (WE) a commercially sustainable technology. When powered by renewable sources, WE is the “greenest” way to produce H2 as it is does not generate any direct carbon emission. Thus, it could give a significant contribution to the prevention of pollution and reduction of greenhouse gas (GHG)-emissions, on which climate neutrality is built, and meet the needs of the 733 million people still living without access to electricity.
The project is focused on the development and engineering of novel nanocatalysts based on high-entropy materials (HEMs) to be used for green hydrogen production via alkaline WE in an electrolyser employing a polymeric anion exchange membrane (AEM) as electrolyte. Its focus is coherent with the emerging strategic theme “Sustainability and protection of natural resources” related to the objectives of Clusters No. 5 (Climate, Energy and Mobility), Sub-Cluster No. 4 (Efficient and sustainable use of energy, accessible and safe for all is ensured thanks to a clean energy system and a just transition) of the Research and Innovation European Framework Programme (RIEFP) 2021-27, as well as with the 7th (Affordable and clean energy) and 13th (Climate action) goals for Sustainable Development of the 2030 Agenda adopted in 2015 by the United Nations.

Planned activities :
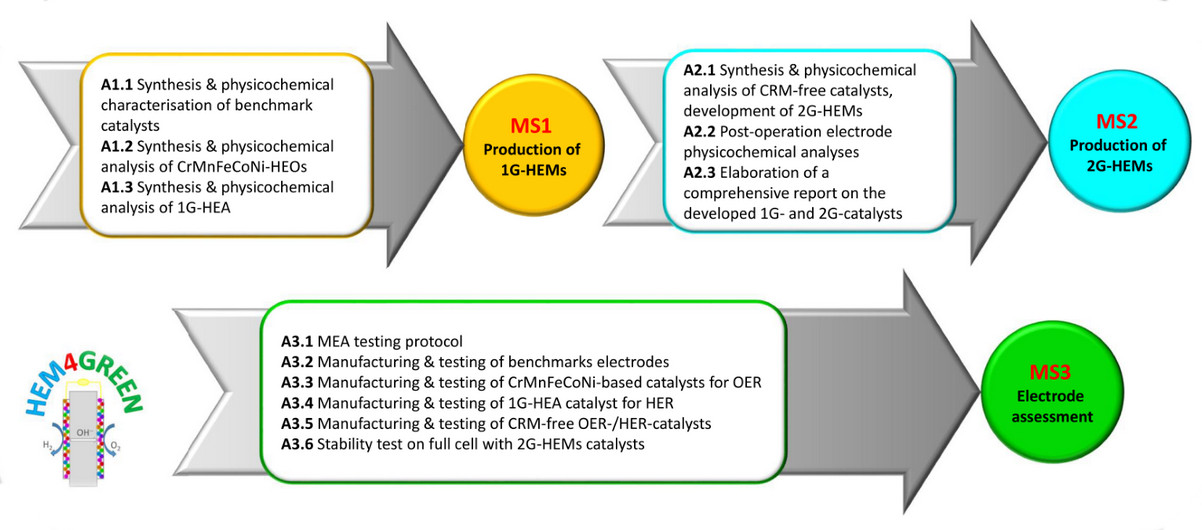
- MS1 (Production of 1G-HEMs) will be reached through the activities carried out by UNIRC:
- A1.1 Synthesis and physicochemical characterisation of benchmark catalysts
- A1.2 Synthesis and physicochemical analysis of CrMnFeCoNi-HEOs
- A1.3 Synthesis and physicochemical analysis of 1G-HEA.
- MS2 (Production of 2G-HEMs) will be reached through the activities carried out by UNIRC:
- A2.1 Synthesis and physicochemical analysis of CRM-free catalysts, development of 2G-HEMs
- A2.2 Post-operation electrode physicochemical analyses (conducted together with CNR-ITAE)
- A2.3 Elaboration of a comprehensive report on the developed 1G- and 2G-catalysts.
- MS3 (Electrode assessment) will be reached through the activities carried out by CNR-ITAE:
- A3.1 MEA testing protocol
- A3.2 Manufacturing and testing of benchmarks electrodes
- A3.3 Manufacturing and testing of CrMnFeCoNi-based catalysts for OER
- A3.4 Manufacturing and testing of 1G-HEA catalyst for HER
- A3.5 Manufacturing and testing of CRM-free OER-/HER-catalysts
- A3.6 Stability test on full cell with 2G-HEMs catalysts.
Dissemination activities :
High-Entropy Materials as cost-effective catalysts in anion exchange membrane water electrolysis for GREen hydrogEN production (HEM4GREEN project): Overview and preliminary results
9th Symposium on Hydrogen, Fuel Cells and Advanced Batteries -
Milazzo (ME), Italy, 30 June – 3 July 2024
Oral presentation by S. Santangelo
Forthcoming events
- Electrospun spinel-structured high-entropy oxides as electrocatalysts for alkaline oxygen evolution reaction
247th ECS Meeting -
Montreal (CA), 18–22 May 2025
Oral presentation by S. Santangelo
- Green hydrogen production: new nanostructured materials as electrocatalysts for anion exchange membrane water electrolyzer
247th ECS Meeting -
Montreal (CA), 18–22 May 2025
Oral presentation by C. Alessandrello
- Green hydrogen production from alkaline water electrolysis by high-entropy materials
ICSET 2025, International Congress on Sustainable Energy and related Technologies -
Lipari (ME), Italy, 22–26 June 2025
Presentation by S. Campagna-Zignani
- Nickel-based electrocatalysts for alkaline water electrolysis for green H2 production
EMCEI 2025, 7th Euro-Mediterranean Conference for Environmental Integration -
Reggio Calabria, Italy, 23–26 June 2025
Oral presentation by C. Alessandrello
